PRODUCTS
Our product is the world’s first approved microbiome-based therapy for the treatment of recurrent and refractory Clostridioides difficile infection.
BiomeBank is now leveraging the real-world, human data collected from our existing therapies and using our cutting-edge ConsortiomeTM platform to engineer a pipeline of novel, co-cultured microbial therapies.
An Overview
Our current product is the first microbiome-based therapy to be approved as a biological in the world. The product is donor derived available in a frozen syringe-based formulation for colonoscopy and enema delivery. We are reformulating the product into a capsule and anticipate the encapsulated product to be available through hospitals and pharmacies in the near future.
The product is manufactured in a purpose built Australian GMP facility, following rigorous and quality-controlled screening, and manufacturing processes. It is currently supplied to hospitals and clinics throughout Australia and can be administered by healthcare professionals.
MEDIA RELEASE
BiomeBank announces world first regulatory approval for donor derived microbiome drug
The Evidence
Donor derived Faecal Microbiota Transplantation (FMT) is a highly effective therapy for the treatment of C.difficle infection; the most common cause of health care associated diarrhoea. Multiple randomised controlled trials and meta-analyses of these studies have demonstrated the superiority of FMT over antibiotic therapy alone for the treatment of recurrent C.difficile infection.
While FMT is now the standard of care for recurrent C.difficile infection in most international guidelines, evidence is emerging for its use in other diseases. BiomeBank’s co-founders Dr Sam Costello and Dr Rob Bryant have been pioneers in this field and BiomeBank is facilitating a number of clinical trials in a range of diseases with collaborating organisations.
Current Treatment (Antibiotics) vs Faecal Microbial Transplantation (FMT)
Antibiotic Treatment
FMT
The Development Pipeline
BiomeBank is now leveraging the real-world, human data collected from these therapies as well as employing our cutting-edge ConsortiomeTM platform to engineer and test a pipeline of novel, co-cultured microbial therapies.
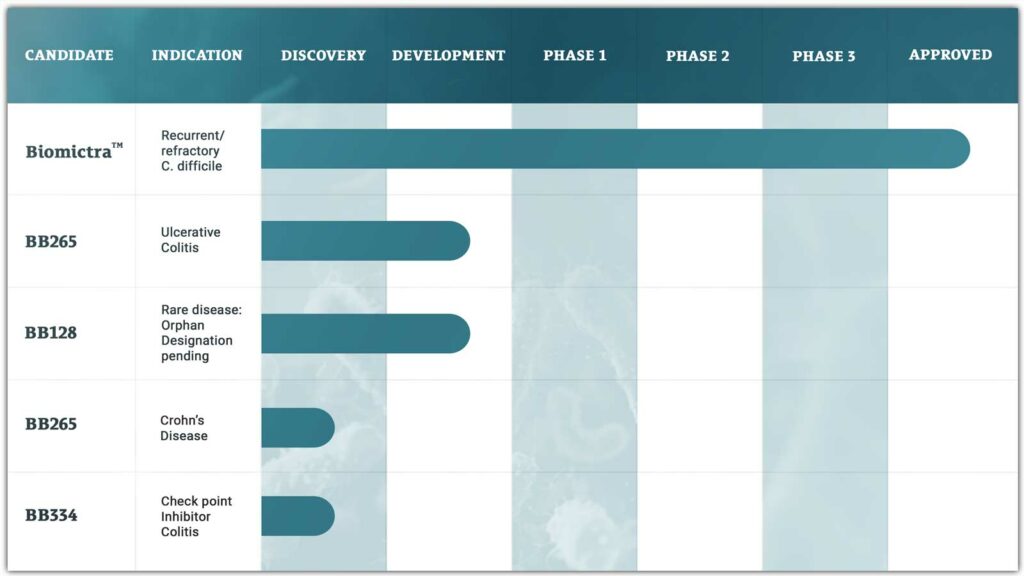
The Potential Drug Candidates
• BB265
BB265 is a rationally designed cultured microbiome therapy engineered for the treatment of ulcerative colitis. BB265 was developed using BiomeBank’s Consortiome platform and data from a discovery trial using BiomictraTM (donor derived) therapy in patients with active ulcerative colitis. BB265 is scheduled to enter a phase 1b/2a study in 2025.
• BB128
BB128 is a rationally designed cultured microbiome therapy engineered for the treatment of an orphan disease indication. A discovery trial in this orphan disease indication is underway using BiomictraTM.
